NMR Without Solvents – Biodiesel Production Process
FAME Product and Glycerol-Methanol By-Product
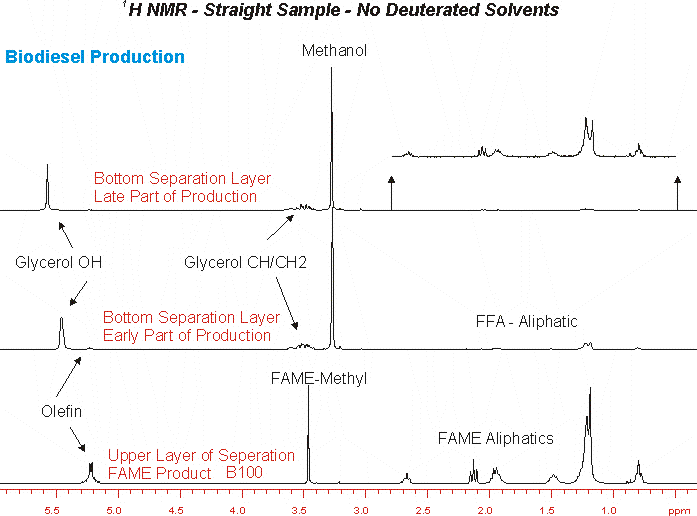
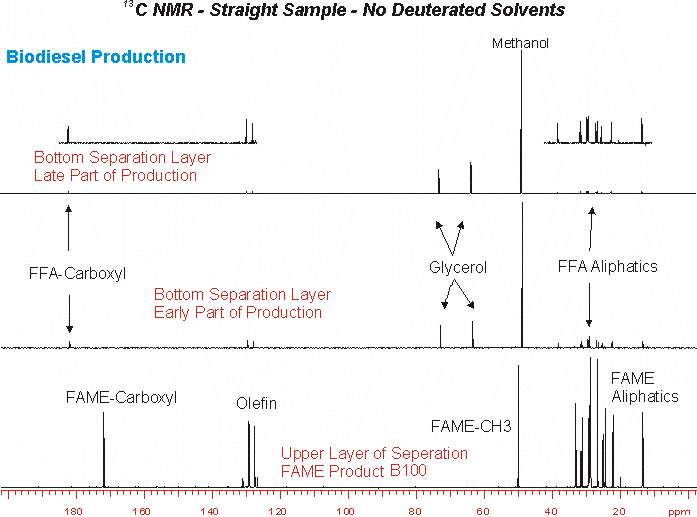
1H and 13C NMR is typically obtained using deuterated NMR solvents to lock the field during acquisition. In some cases the use of these solvents is problematic as it prevents observation of solublized phases present in the sample. As an example we show here the NMR data obtained on a biodiesel production process. One of the major issues with the FAME product is the presence of glycerol in the product. NMR analysis is usually performed by dissolving the FAME in CDCl3 in which glycerol is completely insoluble. Thus NMR analysis performed in this way does not allow analysis of residual glycerol content. However, if the FAME is run neat this issue does not arise.
Another analysis of enormous interest from the process control standpoint is the analysis of the glycerol/methanol phase. This phase contains considerable free fatty acids as well as the glycerol by product and excess methanol from the transesterification process. The three components are readily observed by 1H and 13C NMR, and 23Na can be used to observe NaOH content in the phase. Finally the shift and shape of the observed OH resonance can yield information on the pH of the glycerol phase. Typically this analysis is done in DMSO-d6.
Below are some examples of NMR obtained without a deuterated solvent:
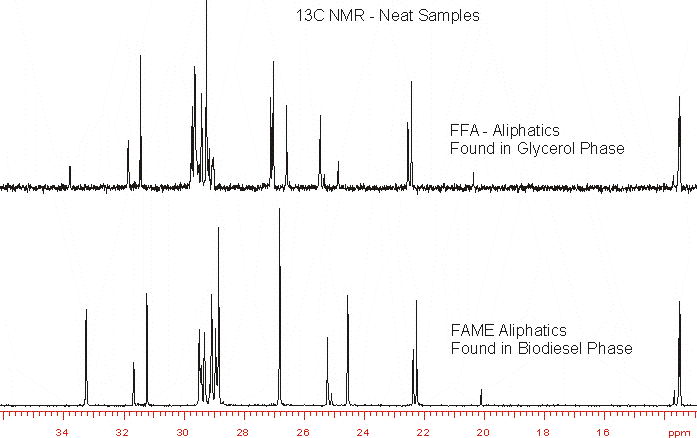
Difference in aliphatic carbon distribution between FAME phase and Free Fatty Acids (FFA) found in the glycerol – methanol phase.
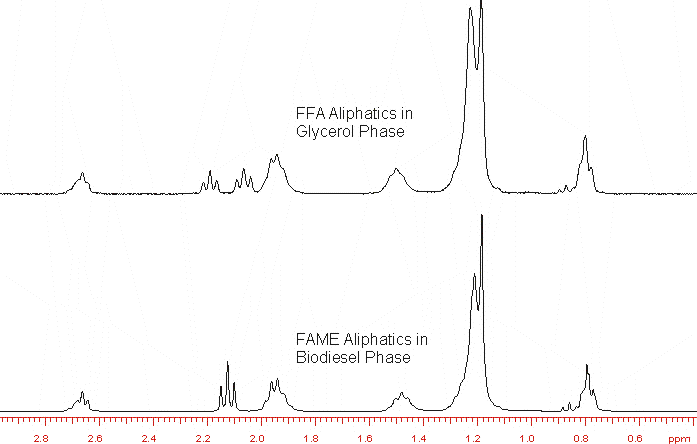
1H NMR of aliphatic component found in the FAME phase as well as the FFA in the glycerol phase.
Contact John Edwards if you are interested in NMR analysis of biodiesel process samples.